
How Covid-19 has been impacting clinical research activities in Brazil
Learn more about steps regarding the research and development of new treatments
Even before the pandemic, the pharmaceutical industry had already been putting a lot of effort into the research and development of drugs and vaccines against Covid-19. This requires clinical trials, which are studies based on scientific guidelines aimed at the discovery or confirmation of effects, therapeutic indications, and/or the identification of adverse reactions to the product being investigated, in order to determine its efficacy and safety in human volunteers.
To understand the main steps between the development of a treatment and its safe and effective application to patients, we have prepared the infographic below:
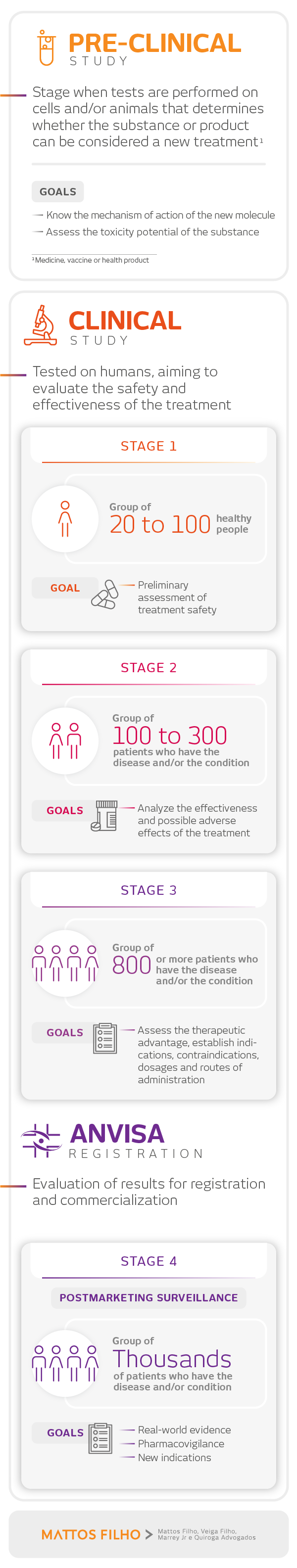
Moreover, clinical studies on human beings require the evaluation of ethical aspects, which must essentially observe factors such as the respect for human dignity and autonomy of the subject of research; risks and benefits of the research (individual and/or collective); prevention of foreseeable harm or predictable losses; and social relevance.
In Brazil, the responsibility for this analysis lies with the Brazilian National Commission of Ethics in Research (Comissão Nacional de Ética em Pesquisa – CONEP), an entity linked to the Brazilian National Health Council (Conselho Nacional de Saúde – CNS), as well as with local Ethics Review Committes (Comitês de Ética em Pesquisa – CEPs) established in hospitals, research centers, and academic institutions. Furthermore, the Brazilian Health Regulatory Agency (ANVISA) may also regulate the subject to some extent, especially if the objective of the clinical trial is a product registration in Brazil for its subsequent commercialization.
Legislative Milestone
Currently, the Brazilian House of Representatives is discussing Bill No. 7,082/2017, which regulates clinical research on human beings and establishes the National System of Ethics in Clinical Research on Human Beings. The Bill has already been approved by the Brazilian Senate and discussed in the Commissions of Science and Technology, Communication and Informatics (CCTCI) and Social Security and Family (CSSF) of the House of Representatives. It is currently awaiting the technical opinion of the Constitution and Justice and Citizenship Commission (CCJC).
This represents an important measure to harmonize ethical principles and guidelines that lead the clinical research development in Brazil, currently regulated only by resolutions of the CNS. The Bill proposes a series of amendments to improve the progress and efficiency of the processes inherent to clinical trials, such as the end of double ethical approvals, in addition to granularity on the criteria for post-trial access to treatment (PTA).
Clinical Research within Covid-19
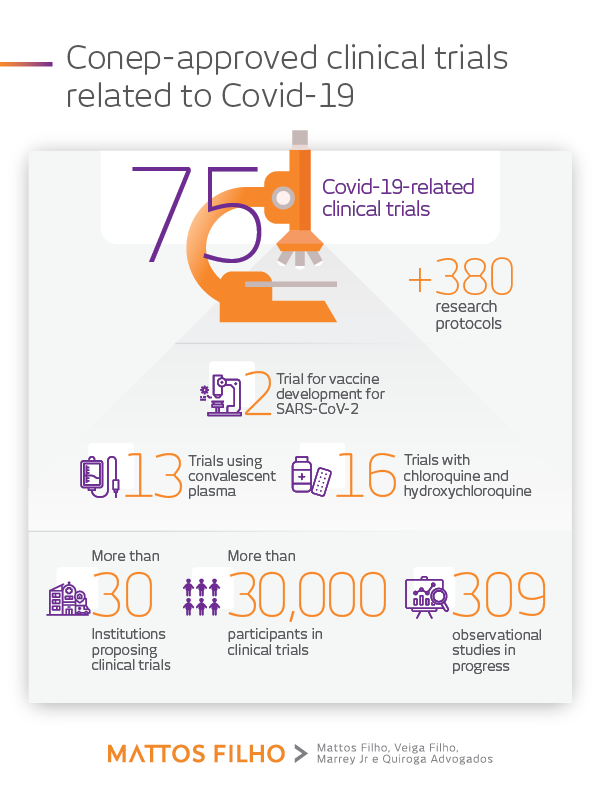
According to CONEP’s data, ethical approval of technical opinions have already been issued for about 384 protocols of scientific research related to coronavirus. The studies have several methodological characteristics, besides great variability in the number of participants of research, from one person (case report), through 1,300 (clinical trial) to the prediction of inclusion of more than 14,000 people (population-based epidemiological study).
Please find below a compilation of the main information published so far by CONEP regarding these studies:
Source: Boletim Ética em Pesquisa da Comissão Nacional de Ética em Pesquisa, Relatório Semanal 21 (Ed. 01/06/2020).
Extraordinary submission rules
Recently, ANVISA published the Board of Executive Officers’ Resolution No. 375, of April 17, 2020, which altered, extraordinarily and temporarily, the submission of clinical trials for validation of medical devices of classes III and IV identified as priorities for use in healthcare services, destined to confront Covid-19.
Due to the public health emergency, the abovementioned trials may be submitted as a Notification in clinical research following the proceeding provided for in the Board of Executive Officers’ Resolution No. 10, of February 20, 2015 (RDC No. 10/2015).
According to RDC No. 10/2015, only medical devices of classes I and II could be subject to the Notification regime, and the other classes would be submitted to a more rigorous proceeding, as they are classified at higher risk for the user.
On Hydroxychloroquine
Regarding technical guidelines for Chloroquine Diphosphate and Hydroxychloroquine prescriptions, the Brazilian Ministry of Health has issued Informative Note No. 5/2020 on technical criteria for chloroquine prescriptions in the public healthcare network for the treatment of hospitalized patients with severe forms of Covid-19.
The medicine will be distributed by the Brazilian Ministry of Health to the State Health Departments, which will send it to the leading hospitals of their respective regions.
The Informative Note highlights that there are national and international clinical studies in progress, evaluating the effectiveness and safety of chloroquine/hydroxychloroquine to be used against Covid-19, as well as other drugs. Therefore, this measure may be modified at any time, depending on new scientific evidence.
Other treatments for patients with Covid-19
Anvisa issued Technical Note No. 3/2020, with guidance to sponsors, centers, and researchers involved in conducting clinical research related to the new coronavirus. According to the Note, Brazil’s epidemiological situation may demand the adjustment of the clinical protocols in progress. See the main orientations:
- Sponsors, in collaboration with clinical investigators and Ethics Committees, may decide whether to discontinue a participant of a clinical trial to preserve its protection. Such decisions should be supported by information and justification on the need for modifications or amendments;
- Triage procedures for Covid-19 in participants of clinical research are not required to be reported to the Agency as an amendment to the protocol;
- The use of alternative processes or modification of existing processes should take into account the approved clinical protocol and the situation of the location or region where the clinical research is being conducted regarding the contingency actions in effect. Amendments to the clinical protocol made exclusively due to Covid-19 countermeasures do not require authorization from ANVISA, but must be submitted in the annual report of the clinical research;
- All efforts to minimize the impacts on the integrity of the clinical research, as well as the inevitable deviations of the clinical protocol concerning Covid-19’s response actions must be documented;
For bioequivalence studies not yet started or already started – but have not yet gone under one or more periods of hospitalization – it is suggested that these periods be postponed.
Plasma as an alternative treatment
Considering recent discussions on the use of convalescent plasma for the treatment of patients diagnosed with Covid-19, Anvisa issued Technical Note No. 19/2020, establishing that if the intended use involves convalescent plasma as a blood component, i.e., plasma from a donor, the submission of a clinical trial for review and prior approval by the Agency is not required.
In such event, the procedure must have its effectiveness approved by the Brazilian Federal Council of Medicine (Conselho Federal de Medicina – CFM), by the Brazilian Ministry of Health, or must be used on an experimental basis, by adhering to the standards provided for research on human beings in Brazil.
In specific situations, considering the public health emergency, the disease severity and the imminent risk condition to the patient’s life, the decision to use convalescent plasma in the treatment of Covid-19 may be made under the accountability of the medical practitioner, who must provide clarification to the patients of the experimental character and the risks involved, with the patients’ or their relatives’ consent.
Another Technical Note, issued by the Brazilian Ministry of Health (Technical Note No. 21/2020), establishes additional technical guidelines regarding the matter.
For more information, contact Mattos Filho’s Life Sciences and Healthcare partners.